paramagnetic vs diamagnetic
An atom could have ten diamagnetic electrons but as long as it also. Paramagnetic materials are slightly attracted by.
 |
| Diamagnetic Vs Paramagnetic Electron Configurations Wize University Chemistry 1 Textbook Wizeprep |
The paramagnetic materials possess the tendency to move from the weaker to the stronger part of.
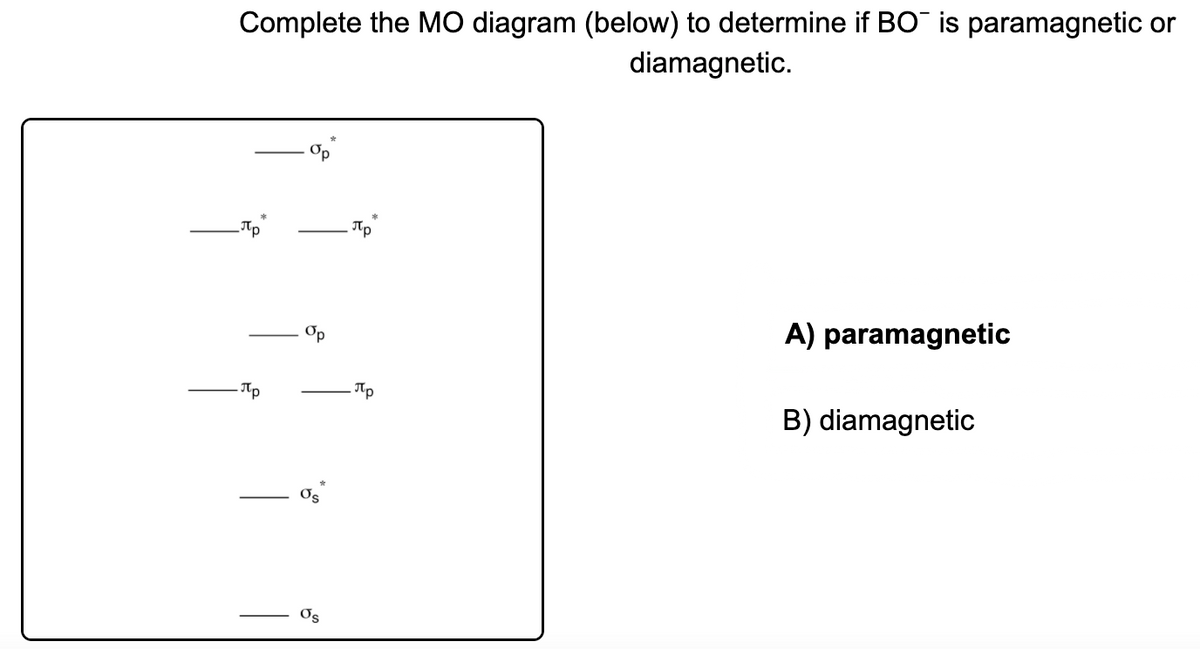
. If you have a situation where you have one electron with spin up and one electron with spin down the. Atoms with all diamagnetic electrons are called diamagnetic atoms. The main difference between Diamagnetism and Paramagnetism is that Diamagnetism produces in resistance to the external magnetic field and goes when the external field is removed. Paramagnetic adjective Having or exhibiting.
Paramagnetic vs Diamagnetic - Paired vs Unpaired Electrons - Electron Configuration 258537 views Jul 12 2016 This chemistry video tutorial focuses on paramagnetism and. 3 if all electrons in the particle are paired then. Ferromagnetic materials will pull themselves towards the north and south poles of magnets. Paramagnetic elements are strongly affected by magnetic fields because their subshells are not completely filled with electrons.
Paramagnetic Substances Substances that get magnetized weakly when placed in an external magnetic field in the same direction as the direction of the externally applied field are known as. To determine whether the elements are. Properties of paramagnetic materials. Repelled by a magnet.
Paramagnetic materials on the other hand will only pull themselves towards one of. Unlike ferromagnetic materials if the magnetic field disappears the magnetic properties that the magnet has will disappear with it. Physics exhibiting paramagnetism Diamagnetic adjective physics Exhibiting diamagnetism. A material that turns at a right angle to the field by producing a magnetic response opposite to the applied field is called diamagnetic material such as silver copper and carbon have.
Therefore a simple rule of thumb is used in chemistry to determine whether a particle atom ion or molecule is paramagnetic or diamagnetic. Whenever two electrons are paired together in an orbital or their total spin is 0 they are diamagnetic electrons. Diamagnetic materials are slightly repelled by a magnetic field and do not retain the magnetic properties when the external field is removed. The properties of these materials differ from Ferro and diamagnetic materials.
Differences between diamagnetic and paramagnetic are as follows. The paramagnetic materials possess the tendency to move from the weaker to the stronger part of. A paramagnetic electron is an unpaired electron. So this situation here is paramagnetic.
What is diamagnetic and paramagnetic. The magnetic fields of the electrons add together. An atom is considered paramagnetic if even one orbital has a net spin. Diamagnetic materials only have paired electrons have a net spin of 0 and are slightly repelled from the applied magnetic fields.
 |
| Ferromagnetic Vs Paramagnetic Vs Diamagnetic What S The Difference Magnets Onemonroe |
 |
| Magnetic Properties Of Materials |
 |
| Contrast Between Diamagnetic Paramagnetic And Ferromagnetic Materials |
 |
| Nicl2 P C2h5 2 C6h5 2 Exhibits Temperature Dependent Magnetic Behaviour Paramagnetic Diamagnetic The Coordination Geometries Of Ni 2 In The Paramagnetic And Diamagnetic States Are Respectively |
 |
| Paramagnetic Vs Diamagnetic Overview Differences Examples Video Lesson Transcript Study Com |
Posting Komentar untuk "paramagnetic vs diamagnetic"